Research
We are fascinated with how mRNAs, the central molecules of post-transcriptional regulation, become spatially organized within cells. Cellular organization of mRNAs is fundamentally important because it creates cellular asymmetries, which polarize cells and help build a body plan of a developing organism. mRNAs often enrich in RNA granules, thus becoming locally concentrated within cells. RNA granules, deemed as the hubs for post-transcriptional regulation, regulate translation and stability of enriched transcripts. They form by phase separation, a process akin to water-and-oil de-mixing. RNA granules form in every cell type and in every organism, are linked with various cellular processes such as stress and cellular differentiation and can even cause disease.
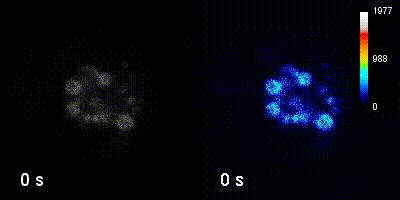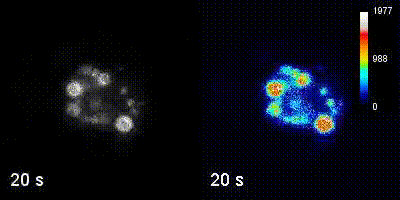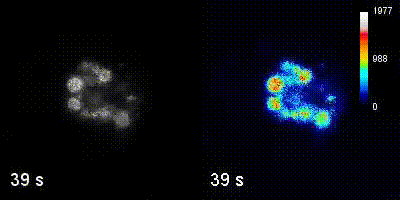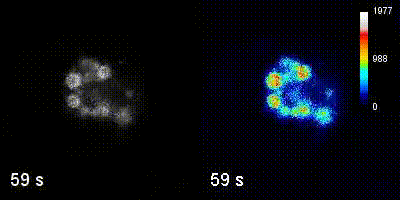
Questions
Diverse RNA granules have been intensely researched. Yet, fundamental questions remain unaddressed:
- Which mRNAs enrich in RNA granules?
- How do mRNAs become enriched in RNA granules?
- How do mRNAs influence granule assembly?
- What happens to the mRNAs once they arrive in RNA granules?
- How are mRNAs regulated in RNA granules?
- How do granule-enriched mRNAs shape the biology of a cell?
- How do granule mRNAs become miss-regulated to drive disease?
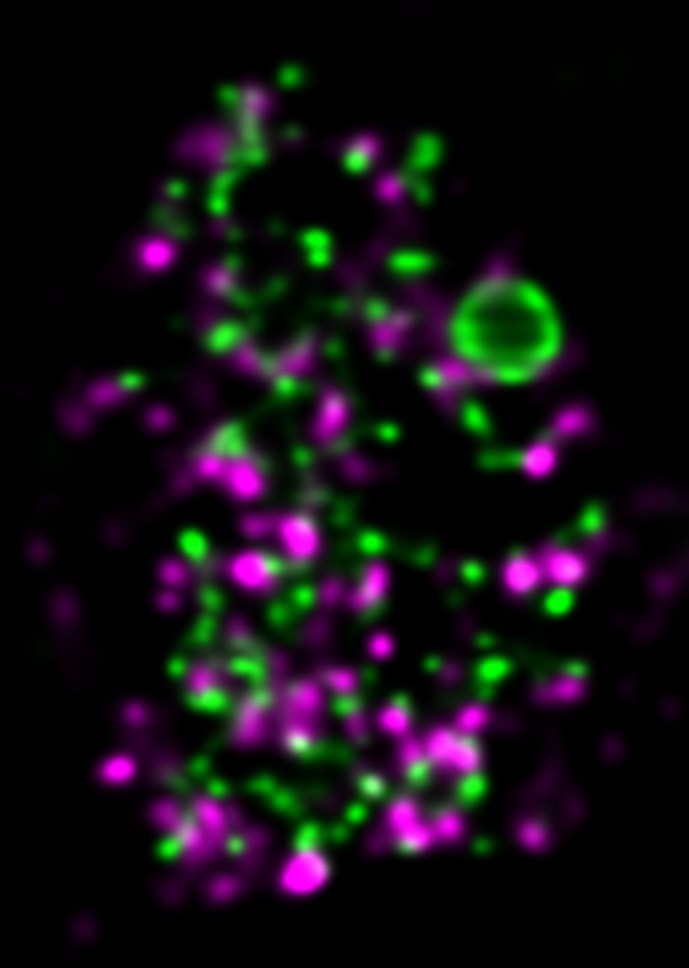
Approach
We employ quantitative, super-resolution imaging approaches in live and fixed cells and tissues coupled with genetic manipulations to address our questions. Combination of these methods affords us with a unique opportunity to understand post-transcriptional regulation of granule mRNAs in wild type and in mutant conditions. By employing our approaches we uncovered that in the fruit fly Drosophila melanogaster, germ granules, the RNA granules that instruct the formation of germ cells and provide the continuity of the spices, form by phase transition and regulate the division of germ cells, that multiple transcripts enriched in these granules form homotypic mRNA clusters, where these mRNA clusters occupy distinct positions within granules and where the mRNAs self-assemble into clusters in a sequence-independent manner.
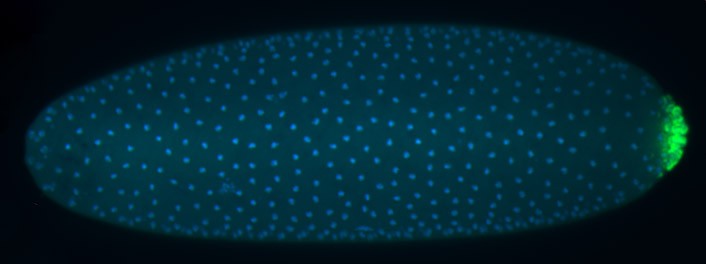
We are now trying to understand how these mRNA clusters form, how they determine their position in granules and decipher their biological function in germline development.
